 Loading... Please wait...
Loading... Please wait...Free Shipping on orders over $500
- Home
- Infection Control
- Prevantics Antiseptic Device Swab
Categories
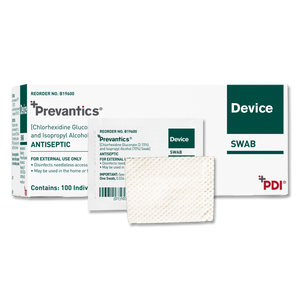
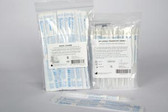
Prevantics Antiseptic Device Swab
Product Description
PDI Prevantics Antiseptic Device Swab
The PDI Prevantics Device Swab is the first and only 3.15% (w/v) Chlorhexidine gluconate (CHG) and 70% (v/v) Isopropyl alcohol (IPA) solution to receive 510k clearance from the US Food and Drug Administration (FDA) for use in disinfecting needleless access sites prior to use.
The PDI Prevantics Device Swab helps target one of the 5 primary sources of CRBSI’s. By implementing protocols for the disinfection of needleless access sites, your facility can help reduce the risk of contamination and improve outcomes.
- The PDI Prevantics Device Swab is made with PDI's unique and proprietary formula of 3.15% Chlorhexidine Gluconate (CHG) and 70% lsopropyl Alcohol and it is the only product specifically tested and indicated by the FDA to disinfect needleless access sites prior to use
- Conveniently designed to help disinfect needleless access sites
- Fast 5 second scrub time and 5 second dry time helps improve medical staff compliance
- Contains 3.15% Chlorhexidine Gluconate and 70% Isopropyl Alcohol
- PDI Prevantics Device Swab achieved a >4.0 log10 reduction versus a leading alcohol impregnated cap1 against 7 tested clinically relevant microorganisms known to cause HAIs*
- 10 Independent clinical studies demonstrating clinical efficacy of Prevantics products*
- The Prevantics Device Swab can be used for both terminal disinfection of a needless access site prior to use as well as in between each access
- Independent studies show that the use of Prevantics Device Swab improves outcomes and improves efficiencies*
- Clinically proven to reduce CLABSI and BSI if used as part of a bundle of evidence-based practices*
- Intuitive, easy-to-use prep-pad design
- 1.0 mL per swap
- Swab Dimensions: 3.125" x 1.125"
- Each swab is individually packaged
- Available in a box of 100 individual swabs and in a box of 20 strips with 8 swabs per strip
- Each box of individual swabs contains 100 swabs, 10 boxes per case (1,000 per case)
- The strips are packaged 8 per strip, 160 total swabs per box, 10 boxes per case
- The strip hangs conveniently on an IV pole for point-of-care accessibility
- Sold by the case
- Manufacturer: PDI Healthcare
Compliant with evidence-based recommendations from the following organizations:
- US Centers for Disease Control and Prevention
- Infusion Nurses Society
- Society for Healthcare Epidemiology of America
- The Joint Commission National Patient Safety Goals
- Infectious Diseases Society of America
- Association for Professionals in Infection Control and Epidemiology
- Association for Vascular Access
*The manufacturer has detailed research and other materials to support this product claim. Contact the manufacturer for more product specifics and research documentation.